Thus there are 5 X 473 grams in the bottle, 2365 grams Divide this weight by the molecular weight of acetic acid (grams/mole) to get the answer MW = 60;Use the number of moles calculated in Step 2 and the molar mass of sucrose from Step 3 to solve for grams 002 mol of C 12 H 22 O 11 × g C 12 H 22 O 11 / 1 mol C 12 H 22 O 11 = 685 g C 12 H 22 O 11Using the values above, if titration requires 102 mmol of NaOH to reach the endpoint, the sample must also contain 102 mmol of acetic acid If the volume of the vinegar used is 805 mL, the molarity of acetic acid is 102 mmol / 805 mL = 0127 M In this experiment, a carefully measured volume of vinegar (V analyte) is placed into a beaker and the mass determined
Pdf Effect Of Recycled Content And Rpet Quality On The Properties Of Pet Bottles Part I Optical And Mechanical Properties
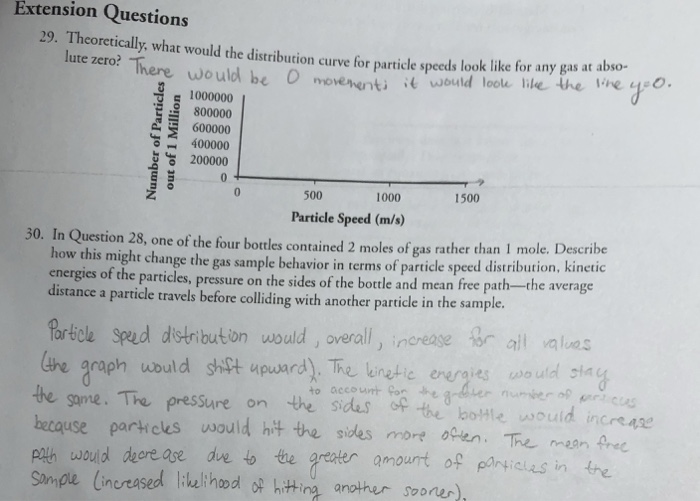
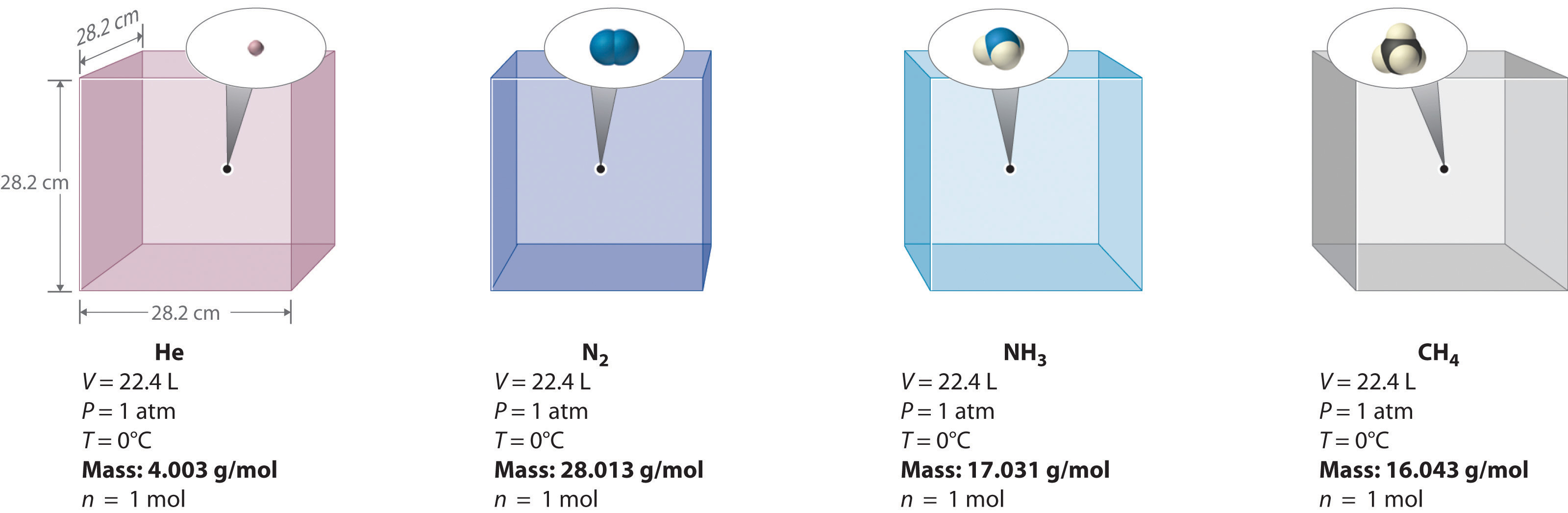
In question 28 one of the four bottles containing 2 moles
In question 28 one of the four bottles containing 2 moles-Question 5 Which of the following correctly represents 360 g of water?Chemistry Q&A Library The chemical 5amino2,3dihydro1,4phthalazinedione, better known as luminol, is used by forensic scientists in analyzing crime scenes for the presence of washedaway blood Luminol is so sensitive that it can detect blood that has been diluted 10,000 times A basic solution of luminol is often sprayed onto surfaces that are suspected of containing minute amounts of blood



How Many Grams Of Carbon Dioxide Gas Is Dissolved In A 1 L Bottle Of Carbonated Water If The Manufacturer Uses A Pressure Of 2 4 Atm In The Bottling Process At 25
A gaseous oxide contains 304% of nitrogen, one molecule of which contains one nitrogen atom The density of the oxide relative to oxygen gas is 1/2 mole C) 3/4 mole D) 2 moles 53 How many gms of copper(at No64) would be displaced from the copper sulphate solution by adding 27 gm of aluminium(at A compound contains 28% nitrogen andOne of the challenges of using the second law of thermodynamics to determine if a process is spontaneous is that we must determine the entropy change for the system and the entropy change for the surroundings An alternative approach involving a new thermodynamic property defined in terms of system properties only was introduced in the late nineteenth century by American mathematician JosiahCalculate its molar mass •One mole SO 2 contains 602 x 10 23 SO 2 molecules, which consist of 602 x 1023 S atoms and 2(602 x 10 23) O atoms •Same for ionic compounds, such as potassium sulfide (K 2 S) Concept 4 The information in a chemical formula
2, shows that its molecules contain one S atom and two O atoms;Question 84 (a) Explain why on addition of 1 mol glucose to 1 litre water the boiling point of water increases (b) Henry's law constant for CO 2 in water is 167 × 10 8 Pa at 298 K Calculate the number of moles of CO 2 in 500 ml of soda water when packed under 253 × 10 5 Pa at the same temperature (CompttConsider a container of fixed volume 250 L We inject into that container 078 moles of N 2 gas at 298 K From the Ideal Gas Law, we can easily calculate the measured pressure of the nitrogen gas to be 0763 atm We now take an identical container of fixed volume 250 L, and we inject into that container 022 moles of O 2 gas at 298K The
Molar mass of solvent (CS 2) = 76g mol1 Question 3 The vapour pressure of pure benzene at a certain temperautre is 0 mm Hg The vapour pressure of a solution containing 2 g of a non¬volatile solute in 78 g of benzene is 195 mm Hg What is the molar mass of the solute?30 In Question 28, one of the four bottles contained 2 moles of gas rather than 1 mole Describe how this might change the gas sample behavior in terms of particle speed distribution, kinetic energies of the particles, pressure on the sides of the bottle and mean free path—the averageGlass milk bottles contain 05 litres of milk When the milk is used up the empty bottles are returned to be reused Glass milk bottles are reused 24 times on average The glass to make new milk bottles is produced when a mixture of sand, limestone, soda and recycled glass is heated to about 1600 °C in a furnace



This Week In Mac Sports 9 15 The Mac Weekly



Bansal Classes Chemistry Study Material For Iit Jee By S Dharmaraj Issuu
10 a 0 ounce bottle of mountain dew contains 770 g of sugar how many calories would a person consume in a 365 day year if they drank one 0 ounce bottle of mountain dew per day?Before investigating the scene, the technician must dilute the luminol solution to a concentration of 300×10−2 The diluted solution is then placed in a spray bottle for application on the desired surfaces The forensic technician at a crime scene has just prepared a luminol stock solution by adding 1g of luminol into a total volume of 750mL of H2OCalculate the pressure exerted by 2 moles of sulphur hexafluoride in a steel vessel of volume 6 dm 3 at 70°C assuming It is an ideal gas Answer We will use the ideal gas equation for this calculation as below P = \(\frac {nRT}{V}\) = 939 atm Question 12 A mixture of gases contains 476 mole of Ne, 074 mole of Ar and 25 mole of Xe



Pdf Mole Concept And Problems Solving Approaches In Life Sciences


Skincell Pro How Does It Remove Mole And Skin In Depth Analysis
C 6 H 6 Dichloroethane, a compound that is often used for dry cleaning, contains carbon, hydrogen, and chlorine It has a molar mass of 99 g/mol Analysis of a sample shows that it contains 243% carbon and 41% hydrogenThere is a clear relationship between O 2 and H 2 O for every one mole of O 2, two moles of H 2 O are produced Therefore, the ratio is one mole of O 2 to two moles of H 2 O, or latex\frac{1\text{ mol O}_2}{2\text{ moles H}_2\text{O}}/latex Assume abundant hydrogen and two moles of O 2, then one can calculateCheck the below NCERT MCQ Questions for Class 9 Science Chapter 3 Atoms and Molecules with Answers Pdf free download MCQ Questions for Class 9 Science with Answers were prepared based on the latest exam pattern We have Provided Atoms and Molecules Class 9 Science MCQs Questions with Answers to help students understand the concept very well



A Bottle Is Heated With Mouth Open To Have A Final Temperature As



Empty Bottle Zelda Wiki
Tdrink 9 a 12 ounce can of a soft drink such as coke classic contains 390 g of fluid ounce 2957 ml what is the mass/volume percent of sugar in coke classic?Calculate its molar mass •One mole SO 2 contains 602 x 10 23 SO 2 molecules, which consist of 602 x 1023 S atoms and 2(602 x 10 23) O atoms •Same for ionic compounds, such as potassium sulfide (K 2 S) Concept 4 The information in a chemical formula(Molar mass of benzene 78 g mol1) Answer 80 g/mol Question 4



The Kinetic Energy Of A Particle In A Sample Can Be Calculated By The Following Course Hero
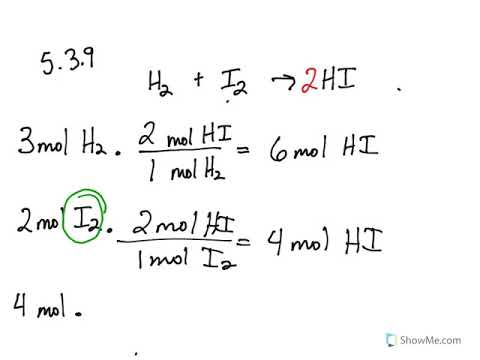


5 3 Calculating Reaction Yields Problems Chemistry Libretexts
A student pours mineral salts into a bottle of cold water Question 28 SURVEY 180 seconds Report question What is the molarity of a solution containing 80 moles of solute in 500 mL of solution?The US Environmental Protection Agency (EPA) places limits on the quantities of toxic substances that may be discharged into the sewer system Limits have been established for a variety of substances, including hexavalent chromium, which is limited to 050 mg/LOne factor to consider since the tablet contains a carbonate, the neutralization reaction produces carbon dioxide Because CO 2 dissolves in water to produce carbonic acid, H 2 CO 3, it can cause your results to be off You will drive off the CO 2 by heating the solution just below boiling for about 5 minutes to alleviate this problem


At What Temperature Are The Helium Particles In The Sample Moving The Fastest On Average 500 C 8 When A Sample Of Gaseous Matter Is Heated Do All Course Hero



The Ideal Gas Law And Kinetic Theory Mcgraw Hill Education Access Engineering
A gaseous oxide contains 304% of nitrogen, one molecule of which contains one nitrogen atom The density of the oxide relative to oxygen gas is 1/2 mole C) 3/4 mole D) 2 moles 53 How many gms of copper(at No64) would be displaced from the copper sulphate solution by adding 27 gm of aluminium(at A compound contains 28% nitrogen andQuestion 1 Calculate the mass percent of benzene (C 6 H 6) and carbon tetrachloride (CCl 4) if 22 g of benzene is dissolved in 122 g of carbon tetrachloride Answer Question 2 Calculate the mole fraction of benzene in a solution containing 30% by mass of it in carbon tetrachlorideFor example, if you want to find the concentration of 10 g of cocoa powder mixed with 12 L of water, you would find the mass of the water using the density formula The density of water is 1,000 g/L, so your equation would read 1,000 g/L = m/(12 L) Multiply each side by 12 L to solve the mass in grams, so m = (12 L)(1,000 g/L) = 1,0 g


Beverages Free Full Text New Trends In Beverage Packaging Systems A Review Html



Doc 117 B P S Xi Chemistry Iit Jee Advanced Study Package 14 15 By S Dharmaraj Issuu
The first two members of the series KH, CaH 2 are ionic hydrides whereas the other members of the series CH 4, C 2 H 6, SiH 4, B 2 H 6, NH 3 are covalent hydrides Question 27 Write chemical equation for the following reactions4 H 2 O leaving mostly NH 4 in the final solution Since only one member of the NH 4 /NH 3 conjugate acidbase pair is left, the solution cannot buffer both base and acid 1 point is earned for the correct response with an acceptable justification (ii) Calculate the final NH 4 in the resulting solution at 25°C moles25 A gas sample contains 01 mole of oxygen and 04 mole of nitrogen If the sample is at standard temperature and pressure, what is the partial pressure due to nitrogen?



Solved Indicate The Answer Choice That Best Completes The Chegg Com



Tattoo Ink Contains Cancer Causing Chemicals So Why Isn T It Regulated Guardian Sustainable Business The Guardian
Then we had to tilt the glass bottle so we could put the 1/2 tablet of alka seltzer in it (by using tweezers) After inserting the tablet, we had to wait for the bottle to stop fizzing or bubbling Then we had to slide the glass piece back underneath the bottle and flip it over We had to repeat the same process for the other 1/2 tablet2 in a soft drink after the bottle is opened and equilibrates at 25 C under a CO 2 partial pressure of 30 10 4 atm Answer 10 10 5 M Calculate the concentration of CO 2 in a soft drink that is bottled with a partial pressure of CO 2 of 40 atm over the liquid at 25 C The Henry's law constant for COMoles, shrews and meadow voles can be similar in appearance Because they look similar and often share the same habitat, you should know how their habits differ so you can identify each species in case it becomes necessary to control them Figure 1 Eastern mole Figure 2 Moles "swim" through soil, often near the ground surface


The Ideal Gas Law And Some Applications Introductory Chemistry 1st Canadian Edition



How Much Water Actually Goes Into Making A Bottle Of Water The Salt Npr
Calculate the pressure exerted by 2 moles of sulphur hexafluoride in a steel vessel of volume 6 dm 3 at 70°C assuming It is an ideal gas Answer We will use the ideal gas equation for this calculation as below P = \(\frac {nRT}{V}\) = 939 atm Question 12 A mixture of gases contains 476 mole of Ne, 074 mole of Ar and 25 mole of Xe2, shows that its molecules contain one S atom and two O atoms;Use this page to learn how to convert between moles and atoms Type in your own numbers in the form to convert the units!



Ncert Chemistry States Of Matter Dronstudy Com



The Kinetic Energy Of A Particle In A Sample Can Be Calculated By The Following Course Hero
You have 25 moles hydrogen gas, 35 moles oxygen gas, and 40 moles nitrogen gas If one balloon is filled with helium and another is filled wit air to the same volume and 28 A student is presented with four bottles con aining different gases All samples are at the same temperature I mole I moleMoles Fe2 in 25 cm3= 5 × 4312 × 104 = 2156 × 103 Moles Fe2 in 250 cm3= 10 × 2156 × 103 = 2156 × 102 Suggest why, in an analysis of an antacid, it is important to test samples from more than one bottle of the antacid Concentrated nitric acid reacts with magnesium to form an oxide of nitrogen which contains 304% by mass›› Quick conversion chart of moles to atom 1 moles to atom = E23 atom 2 moles to atom = 1442E24 atom 3 moles to atom = E24 atom 4 moles to atom = E24 atom 5 moles to atom = 3



Pdf Solutions Manual Jisoo Kim Academia Edu



The Mole Chemistry For Non Majors
One mole of glycine, C 2 H 5 O 2 N, contains 2 moles of carbon, 5 moles of hydrogen, 2 moles of oxygen, and 1 mole of nitrogen The provided mass of glycine (~28 g) is a bit more than onethird the molar mass (~75 g/mol), so we would expect the computed result to be a bit greater than onethird of a mole (~033 mol)Moles EDTA Moles Ca2 in sample Moles Ca2 per liter Grams CaCO3 per liter Water hardness (ppm, mg CaCO3/L Sample ) Average water hardness (ppm CaCO3) _____ Save this value and place in page 4 question 6 of the next experiment, Calcium Analysis by Atomic Absorption, for comparison purposes2, shows that its molecules contain one S atom and two O atoms;



Calculating Partial Pressures Worked Example Video Khan Academy
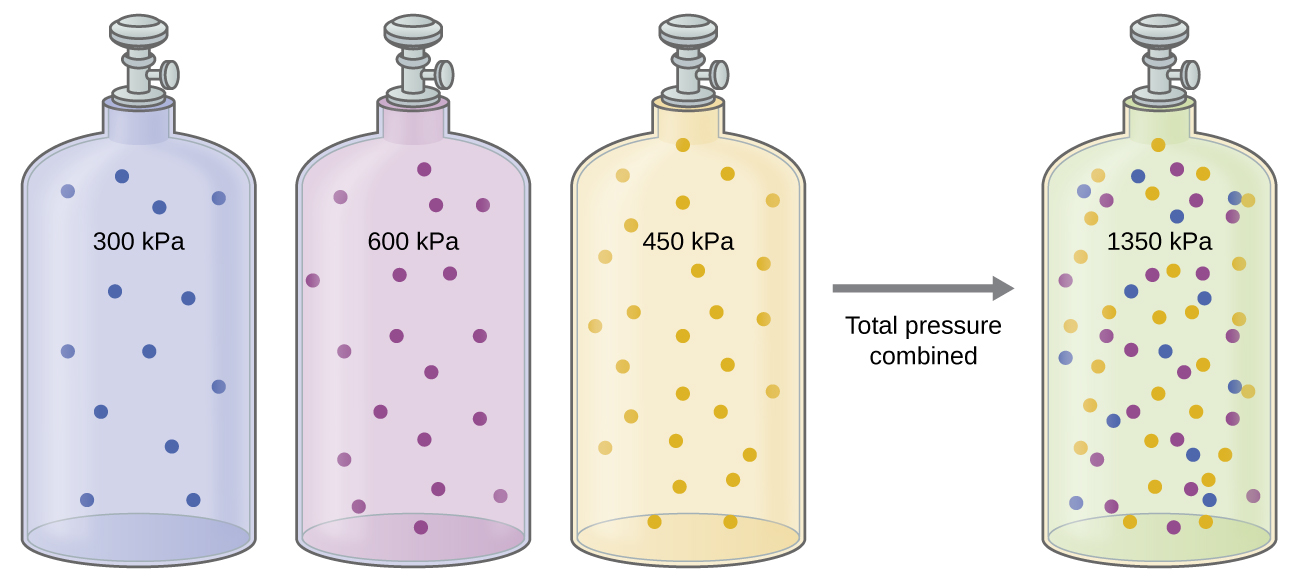


9 3 Stoichiometry Of Gaseous Substances Mixtures And Reactions Chemistry
What volume of a 0M K 2 SO 4 solution contains 57 g of K 2 SO 4?____ 17 A chemical bottle containing K 2 Cr 2 O 7 is 987% pure What mass of Cr is present in 350 g of this chemical?One of the four bottles contains 2 moles of gas rather than 1 mole Describe how this might change the gas sample behavior in terms of particle speed distribution, kinetic energies of the particles, pressure on the sides of the bottle and mean free path—the average distance a particle travels before colliding with another particle in the sample



The Mole Chemistry For Non Majors


Answer Explanations To Previously Released Act Science Test Piqosity Adaptive Learning Student Management App
Calculate its molar mass •One mole SO 2 contains 602 x 10 23 SO 2 molecules, which consist of 602 x 1023 S atoms and 2(602 x 10 23) O atoms •Same for ionic compounds, such as potassium sulfide (K 2 S) Concept 4 The information in a chemical formulaSolution MV = grams / molar mass (x) (1000 L) = 2450 g / g mol¯ 1 x = M to four sig figs, 2498 M If the volume had been specified as 100 L (as it often is in problems like this), the answer would have been 250 M, NOT 25 M1 996 x 1019 moles of copper 2 301 x 10 24 atoms of silver 3 306 x 10 21 atoms of gold 4 167 moles of sulfur 5 grams of iron 6 1 mole of lithium 7 3 moles of oxygen 8 1 x 10 24 atoms of hydrogen 9 241 x 10 24 atoms of oxygen 10 90 moles


Solved Are These Answers Correct I Don T Feel Very Sure Chegg Com
/GettyImages-692027135-419fe3ddc26e4415b356380582c4e5b2.jpg)


How Much Water Is A Mole Of Water
In SI based units it is (48) kg⋅m 2 ⋅mol −1 ⋅K −1 ⋅s −2 Due to this formula people would often refer to the above tool as a "PV nRT calculator" A mole is the amount of substance which contains as many elementary entities as there are atoms in 12 g of carbon12Here's how you can do that As you know, the molarity of a solution tells you the number of moles of solute present for every "1 L" of the solution In order to make the calculations easier, pick a sample of this solution that has exactly "1 L" = 10^3 quad "mL" Use the density of the solution to find the mass of the sample In your case, vinegar is said to have a density of "1005 g mL"^(1A 01 atm b 02 atm c 05 atm d 08 atm e 10 atm 26 A mixture of gases contains 15 moles of oxygen, 30 moles of nitrogen, and 05 mole of water vapor If the



The Ideal Gas Law And Kinetic Theory Mcgraw Hill Education Access Engineering



Thermo Problem Set No 1
In SI based units it is (48) kg⋅m 2 ⋅mol −1 ⋅K −1 ⋅s −2 Due to this formula people would often refer to the above tool as a "PV nRT calculator" A mole is the amount of substance which contains as many elementary entities as there are atoms in 12 g of carbon12(i) 2 moles of H 2 O (ii) moles of water (iii) 6022 × 10 23 molecules of water (iv) 144 × 10 25 molecules of water (a) (i)Bogdan Dreava / EyeEm / Getty Images There are multiple types of flasks One of the most common in a chemistry lab is an Erlenmeyer flask This type of flask has a narrow neck and a flat bottom


Chemistry A Quantitative Science
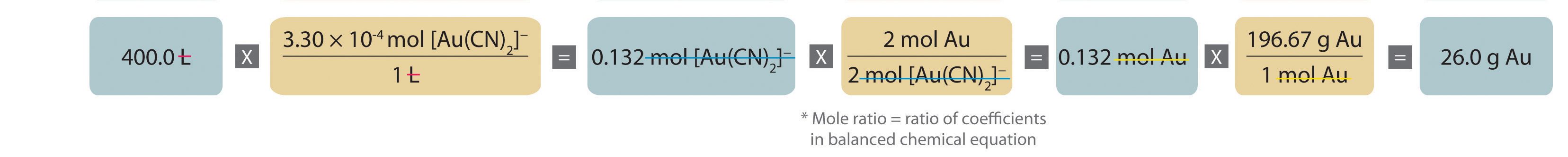


Molarity Solutions And Dilutions M4q6 Uw Madison Chemistry 103 104 Resource Book
6 A laboratory technician discovered four badly−labelled bottles, each containing one pure white solid Each bottle contained a compound of a different Group 2 metal (magnesium, calcium, strontium and barium) Some tests were carried out on the solids or, if the compound was soluble, on the aqueous solution The results are given in theA Calculate the moles of NaOH, Moles = Molarity × Volume of solution= 0146 molL × 00 question_answer Q You have four bottles containing substances in front of youIn Question 28, one of the four bottles contained 2 moles of gas rather than 1 mole Describe how this might change the gas sample behavior in terms of particle speed distribution, kinetic energies of the particles, pressure on the sides of the bottle and mean free path—the average distance a particle travels before colliding with another



Using The Ideal Gas Law To Calculate Number Of Moles Worked Example Video Khan Academy



F 4 Acids And Exercise Titration Chemistry
A 118 g b 1 g c 122 g d 124 g e 126 g ____ 18 Balancing a chemical equation so that it obeys the law of conservation of matter requires a one b two c three d four e fiveAnswer choices 0016 M 16 M 625 M 0063 M s Question 92Question 2 Write the Vander Waals1 equation for one mole of a real gas Answer Question 3 Write Vander Waals equation constants 'a' and 'b' Answer Units for 'a' L 2 atm mol2 Units for 'b' Litre mole1 Question 4 Gie the value of universal gas constant in SI units Answer 124 J K1 mol1 Question 5



Pdf Effect Of Recycled Content And Rpet Quality On The Properties Of Pet Bottles Part I Optical And Mechanical Properties



Amazon Com Nature S Mace Mole Vole Repellent 32oz Castor Oil Concentrate Covers Up To 5 000 Sq Ft Keep Moles And Voles Out Of Your Lawn And Garden Safe To
2 equal 426 moles of CaCl 2?8 A student was given a bottle containing small pieces of scrap iron 4 3 5 28 29 titration 2 37 36 38 titration 3 13 12 14 (d) Use the diagrams to complete the results table titration number 1 2 3 Five moles of Fe2 react with one mole of potassium manganate(VII)Problem #2 What is the molarity of 2450 g of H 2 SO 4 dissolved in 1000 L of solution?



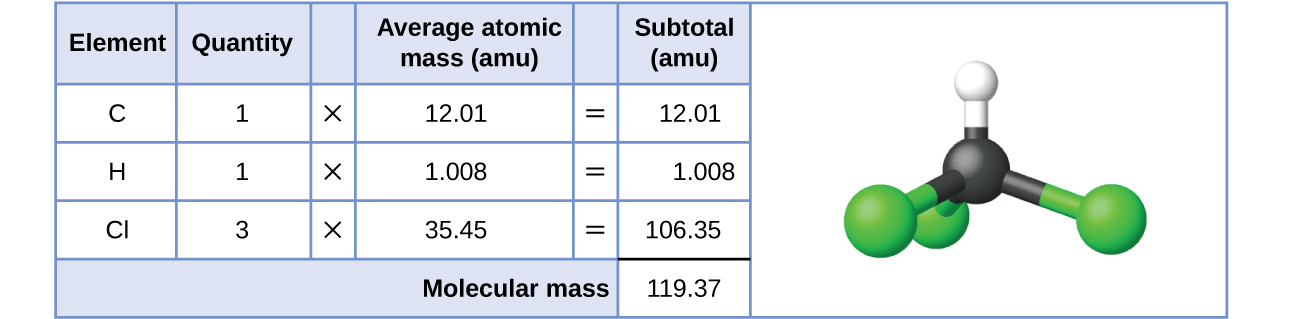
3 1 Formula Mass And The Mole Concept Chemistry Libretexts



Using The Ideal Gas Law To Calculate A Change In Volume Worked Example Video Khan Academy



How Many Grams Of Carbon Dioxide Gas Is Dissolved In A 1 L Bottle Of Carbonated Water If The Manufacturer Uses A Pressure Of 2 4 Atm In The Bottling Process At 25



The Avogadro Constant For The Definition And Realization Of The Mole Guttler 19 Annalen Der Physik Wiley Online Library


The Ideal Gas Law And Some Applications Introductory Chemistry 1st Canadian Edition



Draw Particulate Representations Of Helium Atoms At The Two Different Course Hero


You Have Been Given Four Bottles Marked A B C And D Each Contain


Gases



You Have Been Given Four Bottles Marked A B C And D Each Contain



The Ideal Gas Law And Kinetic Theory Mcgraw Hill Education Access Engineering



Mathematics For The Ib Diploma Analysis And Approaches Sl Draft Copy By Hodder Education Issuu



7 Fill In The Table Below With Your Recorded Data Chegg Com
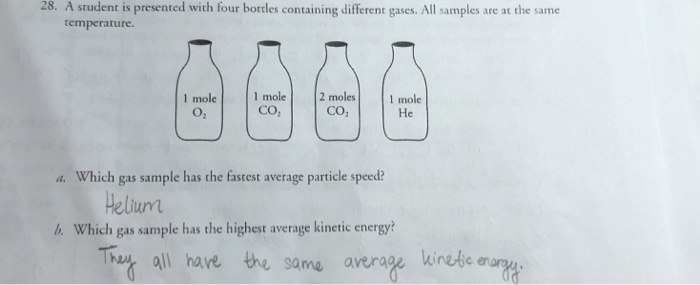


Solved Are These Answers Correct I Don T Feel Very Sure Chegg Com



Amazon Com Liquid Fence Deer Rabbit Repellent Granular 5 Pound Garden Outdoor



The Kinetic Energy Of A Particle In A Sample Can Be Calculated By The Following Course Hero


Formula Mass And The Mole Concept Chemistry
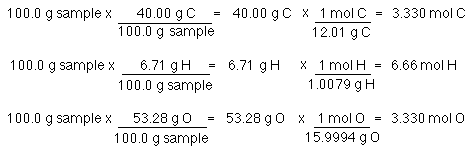


Percent Composition


Chapter 5 Gases


You Have Been Given Four Bottles Marked A B C And D Each Contain



Tracking Viral Misinformation Latest Updates The New York Times



Draw Particulate Representations Of Helium Atoms At The Two Different Course Hero



Thermodynamics Problems



0 件のコメント:
コメントを投稿